- Build a platform technology for smarter antibodies.
- Improve antibodies for current medical targets.
- Enable antibodies for currently untreatable medical targets.
- Leverage revolutionary advances in synthetic biology and AI.
Biocognon’s mission is delivering technology that will transform the development and application range of therapeutic and diagnostic antibodies. Our focus is building a novel combinatorial yeast screening platform for discovery of Nanobodies™, small protein domains that can replace the native molecular recognition components of human antibodies. Robust, readily engineered Nanobodies™ enable the advancement of medicine by providing diverse target-binding specificities that are difficult and sometimes impossible to generate by the human immune system. Biocognon’s combinatorial discovery technology utilizes patented fluorogenic molecular biosensors integrated into a yeast dual library platform that surface displays Nanobodies™ and captures folded ectodomains of cognate antigens secreted from this eukaryote. Our use of synthetic biology for the granular design of both Nanobodies™ and target antigens opens the door to powerful applications of Artificial Intelligence.
Natural human IgG antibodies rely on two-peptide protein domains (termed VH-VL) to recognize and bind medically important target proteins. Remarkably, scientists have discovered that camelids (camels, llamas and relatives) harbor antibodies with single-peptide (VHH) protein domains that support essentially the same recognition and binding capabilities. Camelid VHH, aka Nanobodies™, are half the size and much simpler to engineer. Recent years have seen an explosive increase in the use of Nanobodies™ in recombinant immunoreagents of diverse formats and mechanisms of action. Nanobodies™ can modify the canonical IgG framework to confer chimeric, biparatopic, bispecific and multispecific binding activities not found in natural antibodies. Antibodies exert their therapeutic effects in many ways, including activating or blocking receptors, downregulating receptor expression, blocking ligands, depleting target cells or soluble antigens, and targeting delivery of conjugated toxins or radionuclides. Improved binding specificities conferred by Nanobodies™ promise to extend these applications of antibodies in unanticipated ways and to expand their scope to currently undruggable targets.
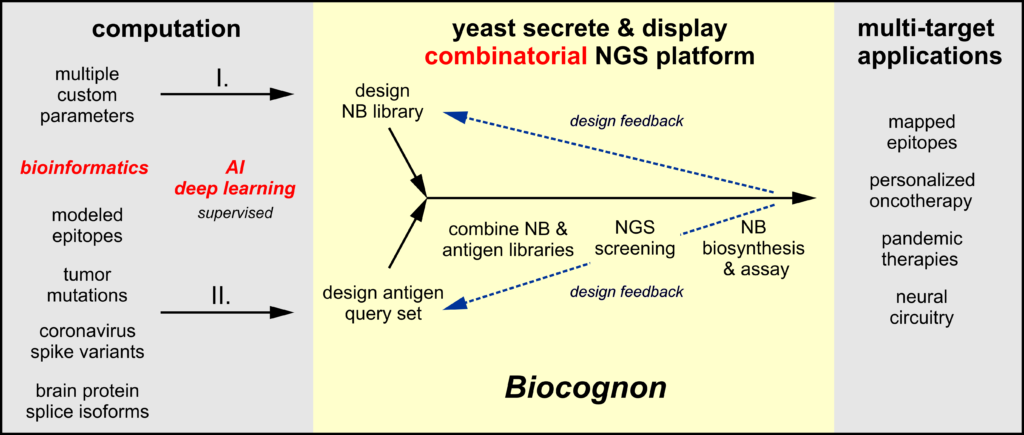
Biocognon’s molecular recognition platform is a bridge technology that directly links genetic data to the creation of antibodies able to discriminate among closely related targets. Bioinformatics can be computationally applied on a massive scale using machine learning to genetic data of many kinds. Nanobody™ libraries (I.) may be customized─prior to screening─for desirable pharmaceutical properties such as diversity, humanization, stability and structure/composition of the molecular recognition loops (CDRs). Small libraries of related target antigens (II.) may be designed as query sets that focus the screen on recognition attributes crucial to the therapeutic or diagnostic of interest. Query sets may be comprised of independent biological entities (mutants, viral variants, isoforms) or may be designed as probes of a single high value therapeutic target to identify or delimit the binding mode (epitopes). Next generation sequencing provides Nanobody™/antigen cross-binding information, and the binding properties of select candidates may easily be quantitatively confirmed by standard in vitro assays using these proteins secreted from yeast. These functional data may then be used in a feed-back loop to design improved Nanobody™ libraries and antigen query sets.
Unlike current Nanobody™ or IgG discovery technologies, Biocognon’s approach utilizes multiple antigens encoded as DNA rather than a single antigen in the form of purified protein. Our combinatorial platform screens fused Nanobody™/antigen libraries in a ‘single pot’ procedure that delivers next generation sequencing output that can be readily analyzed to compare the binding of all Nanobody™/antigen pairs defined in the query set. This combinatorial approach differs fundamentally from current massively parallel screening technologies where the requirement for purified antigen makes comparative analyses of the binding of Nanobodies™ or IgG variants to multiple antigens much more resource-intensive.
Biocognon’s platform is designed to rapidly and inexpensively convert genetic information into antibodies and derivatives that are highly specific for protein antigens. Many therapeutic targets vary in composition over time (tumor neoantigens, pandemic viruses), whereas other targets are inherently and dynamically complex (protein/protein ligand pairs). In both cases, the best therapeutics may be not be single ‘magic bullets’ but instead multiple bullets fired sequentially or simultaneously. Antibodies are ideal for this.